At NDSU, Alyssa Hodges ’21, ’23, ’26 discovered a community ready to support her in ways she hadn’t expected.
Read More20 Years of Front-line Research
Peter Iwen '76 is the recipient of the 2023 Alumni Achievement Award, which recognizes alumni who have attained outstanding professional accomplishments.
Story by Nicole Thom-Arens | March 21, 2023
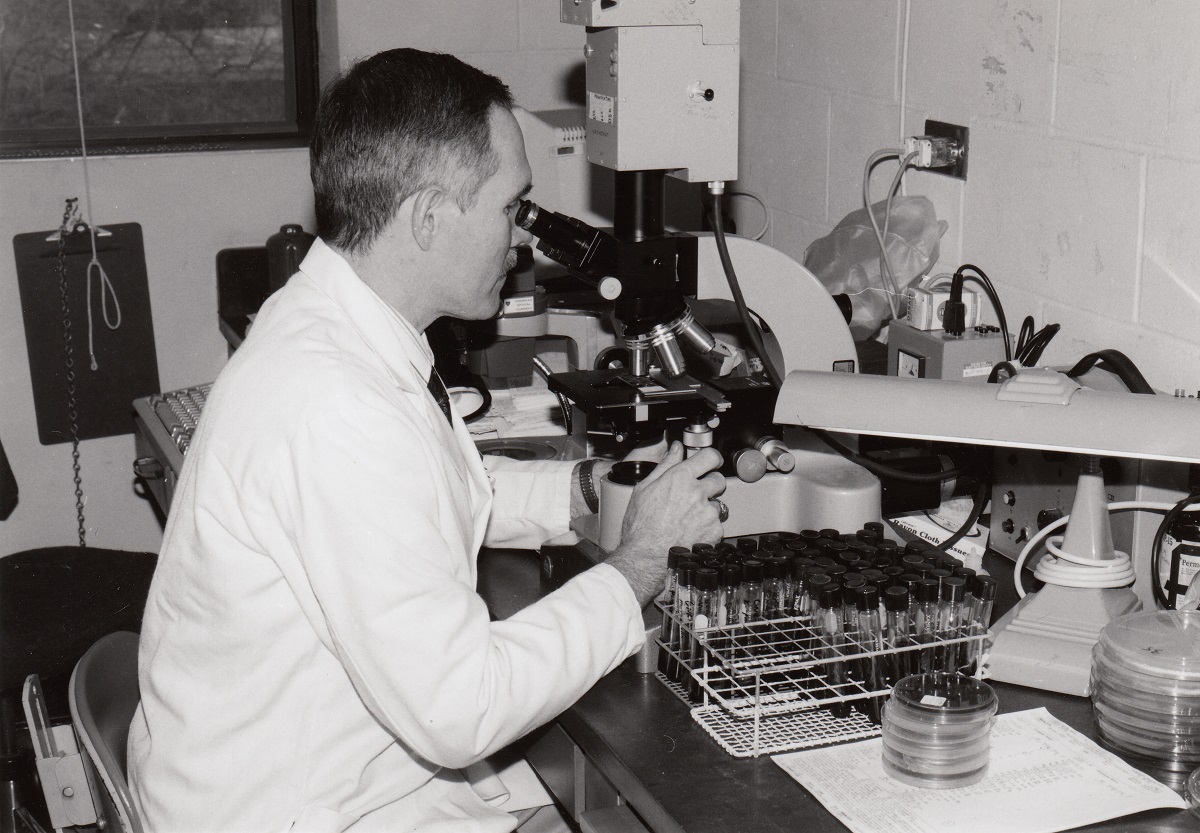
Peter Iwen ’76 grew up in Arthur, North Dakota. His dad, Bert, managed Arthur Drug, and his mom, Joanne, was a registered nurse at the Good Samaritan Home. His parents’ occupations, and the way he saw them meet the needs of the community, led Peter to pursue a career in the sciences. Since graduating from NDSU with a degree in bacteriology, Peter has been on the front line of research and diagnostic testing for some of the world’s most dangerous pathogens of the past 20 years.
Peter launched his career at the University of Nebraska Medical Center (UNMC) in Omaha, Nebraska. His first job was as a researcher studying human fungal pathogens. From there, his supervisor encouraged him to pursue a Master of Science degree, and years later, a new chair of the department of pathology and microbiology encouraged Peter to pursue a Ph.D. In 2001, Peter presented his thesis defense just days after 9/11.
“About a month after 9/11, there were concerns of anthrax spore contamination at the post offices in Washington, D.C., which resulted in a national biothreat release alert,” Peter recalled. “My boss asked me to coordinate the high-volume testing of potentially contaminated specimens from Nebraska sources that occurred as a result of this scare.”
Currently, there are about 80,000 people in the U.S. certified by the U.S. Department of Justice to work with anthrax spores and other biological agents and toxins that pose a severe threat to public health and safety. Peter’s DOJ certification number is 265 — evidence of his entry into this research at the ground level and the number of years he’s specialized in this area of work.
Following graduation with his Ph.D., Peter became the assistant director for the Nebraska Public Health Laboratory (NPHL) and the first campus biosafety officer, where his responsibility was to develop biosafety and biosecurity programs on campus to support research. He also oversaw the high-level containment laboratories located on campus.
In 2014, during the Ebola pandemic in Africa, Peter’s laboratory staff became the first and only state public health laboratory in the U.S. to handle specimens that contained viable Ebola virus to manage infected patients.
“Three infected patients were expatriated to our patient care biocontainment unit at Nebraska Medicine, the campus academic hospital, in which my laboratory personnel were asked to provide laboratory support to care for these patients due to their training in high-level containment,” Peter said. “Following our experiences to support these patients, laboratorians from both national and international locations wanted to know how we safely handled these infected specimens. To share our experiences, we wrote papers to describe procedures, provided training, and collaborated with the CDC to help design protocols. We became recognized as experts to handle emerging special pathogens in a clinical setting, and our work with the CDC continues today.”
In late-February 2020, Peter’s laboratory became involved in COVID-19 diagnostics to support testing of high-risk exposed passengers from the Diamond Princess cruise ship. They were sent to Omaha for quarantine in the National Quarantine Unit.
“Prior to patients arriving in Omaha, we worked with the CDC in Atlanta to verify the test that would identify the causative coronavirus in people,” Peter said. “In the process, we helped the CDC isolate a problem with this earlier test so it could be successfully released to all 65 public health labs in the U.S.”
Peter’s research on COVID-19 testing continued, and early in March 2020, his team at the NPHL developed a highly accurate method for group testing of pooled specimens to conserve reagents, the chemical substances needed to perform COVID-19 tests. Group testing subsequently became a standardized method approved by the FDA used by multiple laboratories to meet the needs for high-number testing of specimens for COVID-19.
Today, Peter is the senior biosafety officer for the UNMC, a professor of microbiology in the College of Medicine, and director of the NPHL.
Share This Story
Related Stories
Glad You’re Here
NDSU students bring companionship and events to residents in memory care at Fargo’s Touchmark retirement community.
Read More
